Milestones of Radiochronobiology Research
1963
Pizzarello et al.
Rats survived 900 roentgens from total body x-ray irradiation (approx. 7.9 Gy) over 130 days when irradiated in the morning. The same dose killed all the animals within 13 days when irradiated at night.
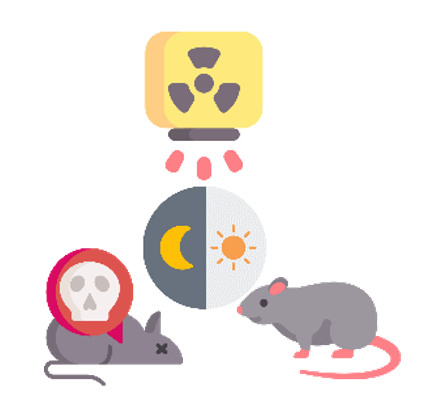
Pizzarello DJ, Witcofski R, Lyons EA. Variations in Survival Time After Whole-Body Radiation at Two Times of the Day. Science. 1963;139:349–365.
1968
Ueno
Endogenous spleen colony-forming cells from X-ray irradiated mice were most radiosensitive (apoptotic) at
2 AM and most radioresistant (proliferating) at 10 PM.
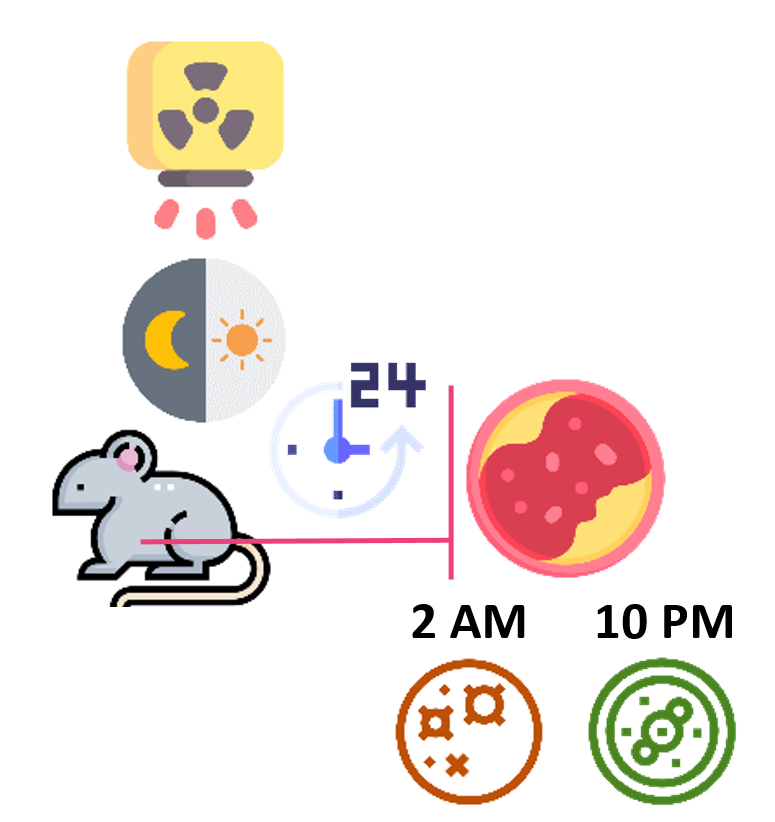
Ueno Y. Diurnal rhythmicity in the sensitivity of haemopoietic cells to whole-body irradiation of Mus musculus. Int J Radiat Biol Relat Stud Phys Chem Med. 1968;14(4):307–312.
1971
Walinder
Iodine-131 uptake in mouse thyroid over 24 hours following intravenous administration varied significantly from 8 AM to 4 PM and was highest at 1 PM.
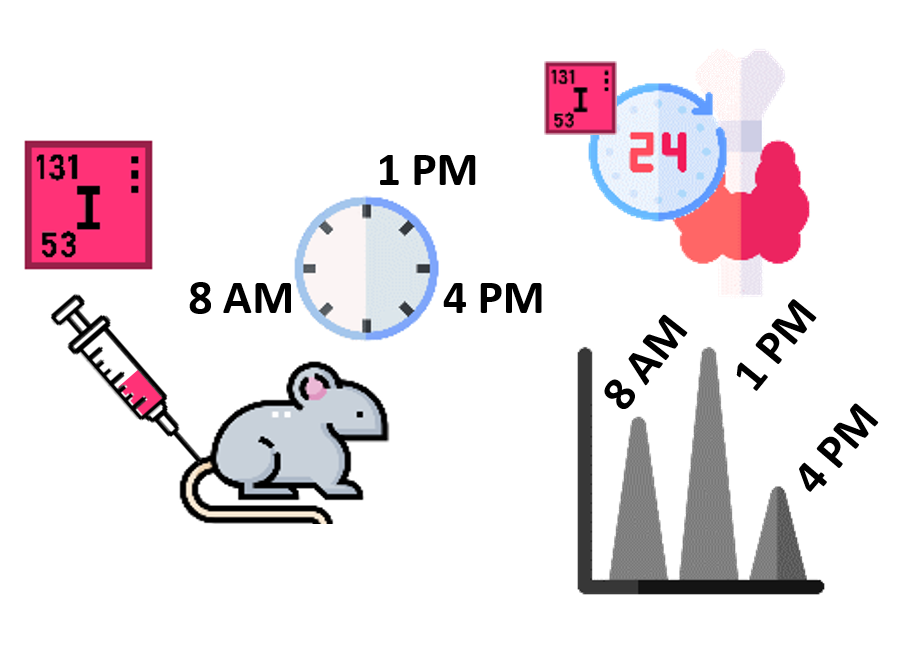
Walinder G. Determination of the 131I dose to the mouse thyroid. Acta radiologica: therapy, physics, biology. 1971;10(6):558–78.
1982
Becciolini et al.
Leucinaminopeptidase enzyme activity showed circadian oscillation.
Enzyme activity was affected by irradiation and normalized faster if rats were irradiated at the end of the dark phase.
Becciolini A, Benucci A, Porciani S, Nardino A, Lanini A. Dipeptidase activity in the small intestine after irradiation at different times of the day. Strahlentherapie. 1982;158(6):368–374.
1983
Duncan et al.
Mice that were whole-body irradiated with 100 rad from gamma radiation (137Cs) at 8 PM showed a decrease in the frequency of apoptotic cells in the duodenum and colon from 12 to 24 h after treatment. Instead, irradiation at 8 AM. resulted in an increase in apoptotic frequency.
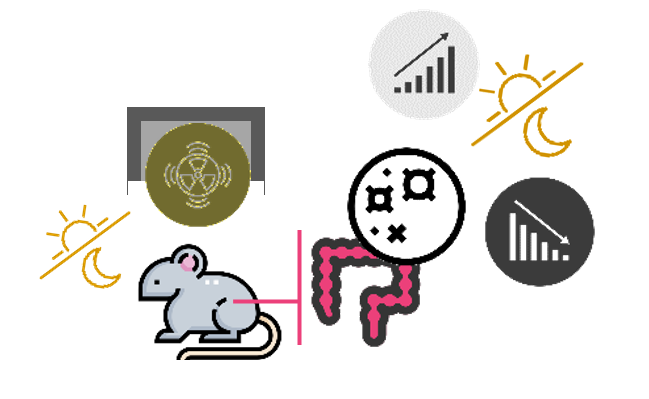
Duncan AM, Ronen A, Blakey DH. Diurnal variation in the response of gamma-ray-induced apoptosis in the mouse intestinal epithelium. Cancer Lett. 1983 Dec;21(2):163–6.
1992
Davydova
The radioresistance of the Central Nervous System (CNS) in rats oscillated during the day as measured by severity and frequency of early transient neurological disorders (ETND) like opisthotonos and convulsions.
ETND were more frequent and severe
in females than in males.
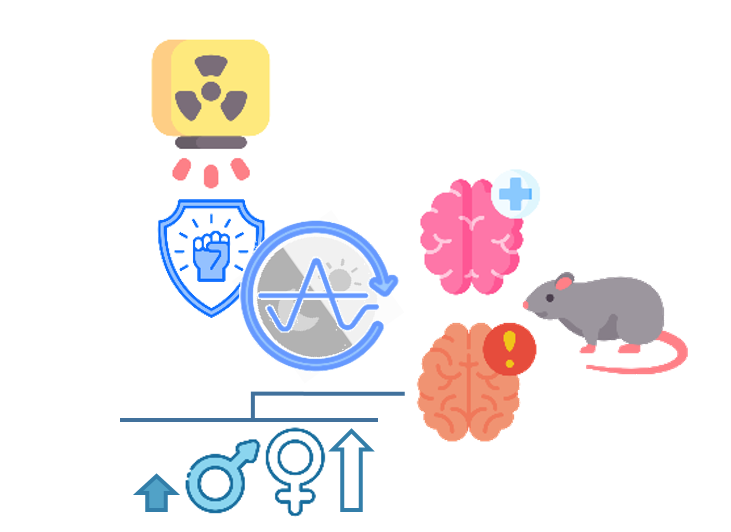
Davydova OE. [The circadian oscillations in the radioresistance of the CNS in female and male rats after craniocaudal gamma radiation] Russian. Radiobiologiia. 1992 Jul-Aug;32(4):596–9.
1994
Hashimoto et al.
The lung tumor incidence in mice after thorax irradiation with 1.25 Gy from X-rays at night compares to 5 Gy at day.
Radiation-induced proliferative activity was significantly higher during the dark phase.
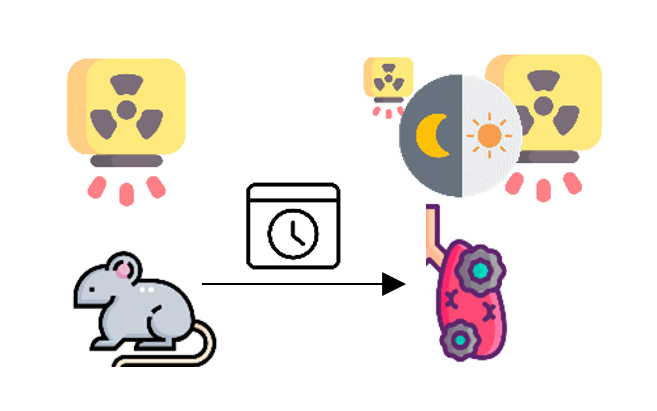
Hashimoto N, Endoh D, Kuwabara M, Satoh H, Sato F. Induction of lung tumors in C3H strain Mus musculus after single or fractionated irradiation with X-rays. J Vet Med Sci. 1994 Jun;56(3):493–8.
1995
Blank et al.
The periodicity of mitotic activity in bone marrow depicts a pronounced tumor-to-healthy circadian differential in rats.
The activity peak occurred shortly after the onset of the dark phase and was similar for intact and tumor-bearing rats.
The circadian rhythm of sarcoma mitotic activity was much smaller and had a different acrophase occurring late in the dark phase.
Correct radiotherapy timing may achieve concomitantly near maximal anti-tumor efficacy and near minimal myelotoxicity.
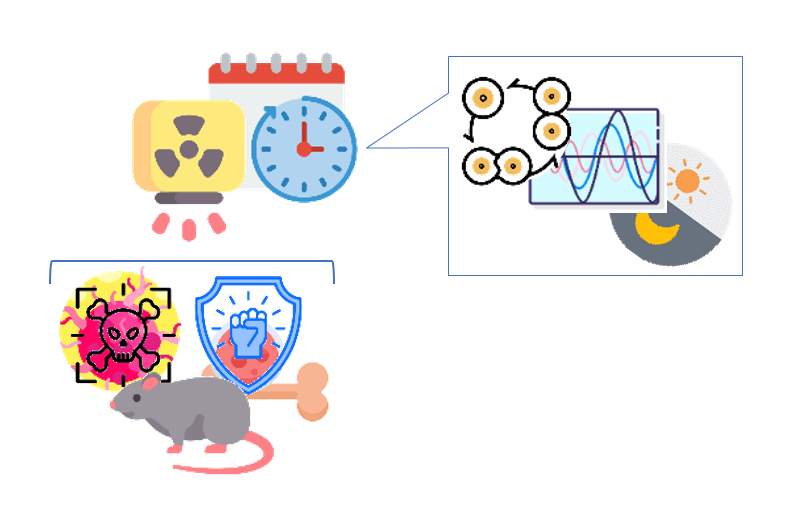
Blank MA, Gushchin VA, Halberg F, Portela A, Cornélissen G. X-irradiation chronosensitivity and circadian rhythmic proliferation in healthy and sarcoma-carrying rats’ bone marrow. In Vivo. 1995 Jul-Aug;9(4):395–400.
2002
Fu et al.
The circadian gene Period2 plays an important role in tumor suppression. Mice deficient in the mPer2 gene were cancer prone. Gamma irradiation of these mice increased tumorigenesis and reduced apoptosis in thymocytes.
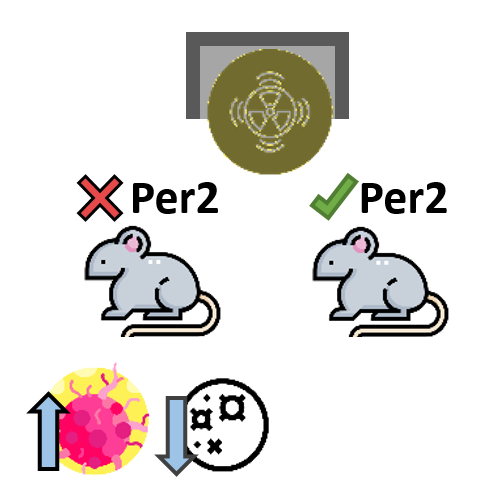
Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002 Oct 4;111(1):41–50.
2008
Oklejewicz et al
Gamma irradiation phase-advanced circadian rhythms in rat fibroblasts in a dose- and time-dependent manner. The underlying mechanism involved ATM-mediated damage signaling.
The effect did not depend on regulation of clock gene expression suggesting that clock resetting is a universal property of DNA damage.
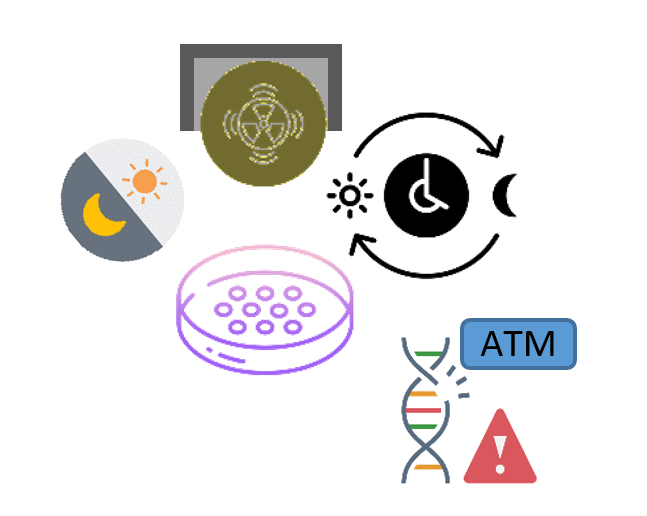
Oklejewicz M, Destici E, Tamanini F, Hut RA, Janssens R, van der Horst GT. Phase resetting of the mammalian circadian clock by DNA damage. Curr Biol. 2008 Feb 26;18(4):286–91.
2015
Langen et al.
The radiation-induced transcriptomic response in various normal tissues 24 hours after intravenous 131I administration in mice at 9 AM, 12 PM, or 3 PM varied strongly with circadian time of treatment.
The data showed that the radiation dose-response is not diurnally constant and that molecular biomarkers for biodosimetry and risk assessment need validation for circadian oscillation of radiation-induced differential gene expression.
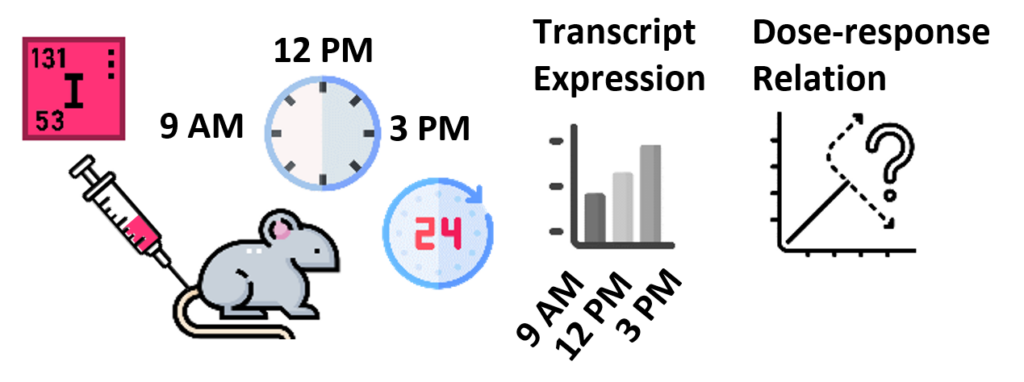
Langen B, Rudqvist N, Parris TZ, Helou K, Forssell-Aronsson E. Circadian rhythm influences genome-wide transcriptional responses to (131)I in a tissue-specific manner in Mus musculus. EJNMMI Res. 2015 Dec;5(1):75.
2020
Dakup et al.a
Circadian clock disruption significantly increased radiodermatitis in mice compared to unirradiated controls.
It also significantly reduced body weight and increased genomic DNA damage in blood cells compared to controls.
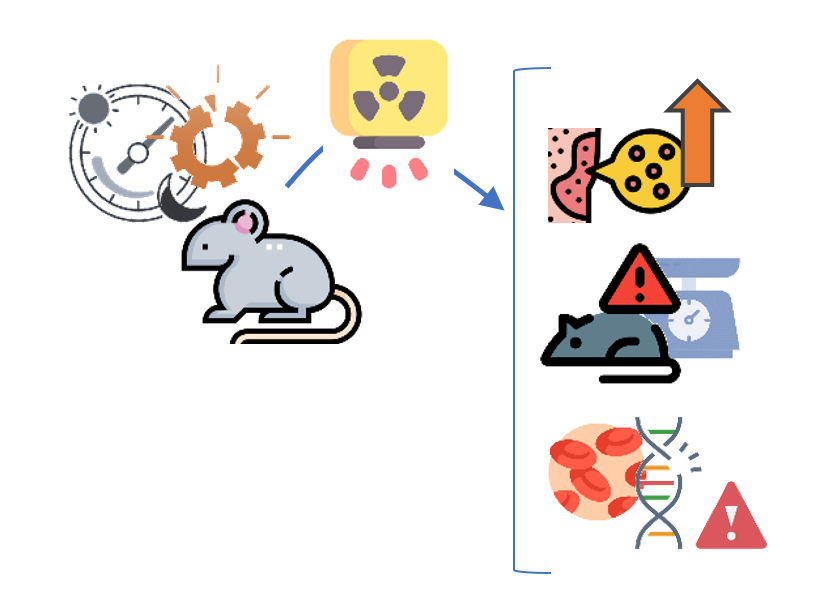
Dakup PP, Porter KI, Gaddameedhi S. The circadian clock protects against acute radiation-induced dermatitis. Toxicol Appl Pharmacol. 2020 Jul 15;399:115040.
2020
Dakup et al.b
Circadian clock disruption significantly exacerbated post-irradiation systolic dysfunction in the heart and increased fibrosis in mice. Circadian-synchronized rat cardiomyocytes (H9c2) and Bmal1 depletion increased radiation-induced
DNA damage and apoptosis.
The circadian clock appears to protect against ionizing radiation toxicity and potentially impacts cancer radiotherapy outcome through radiochronobiological phenomena in DNA damage pathways.
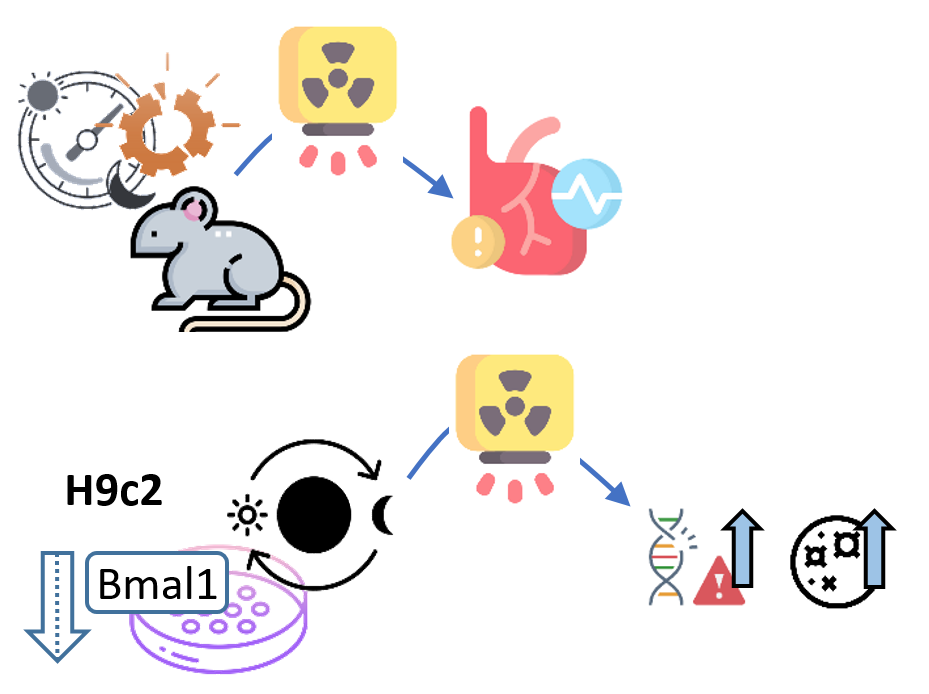
Dakup PP, Porter KI, Gajula RP, Goel PN, Cheng Z, Gaddameedhi S. The circadian clock protects against ionizing radiation-induced cardiotoxicity. FASEB J. 2020 Feb;34(2):3347–3358.
